
Insights+: EMA Marketing Authorization of New Drugs in September 2023
Shots:
-
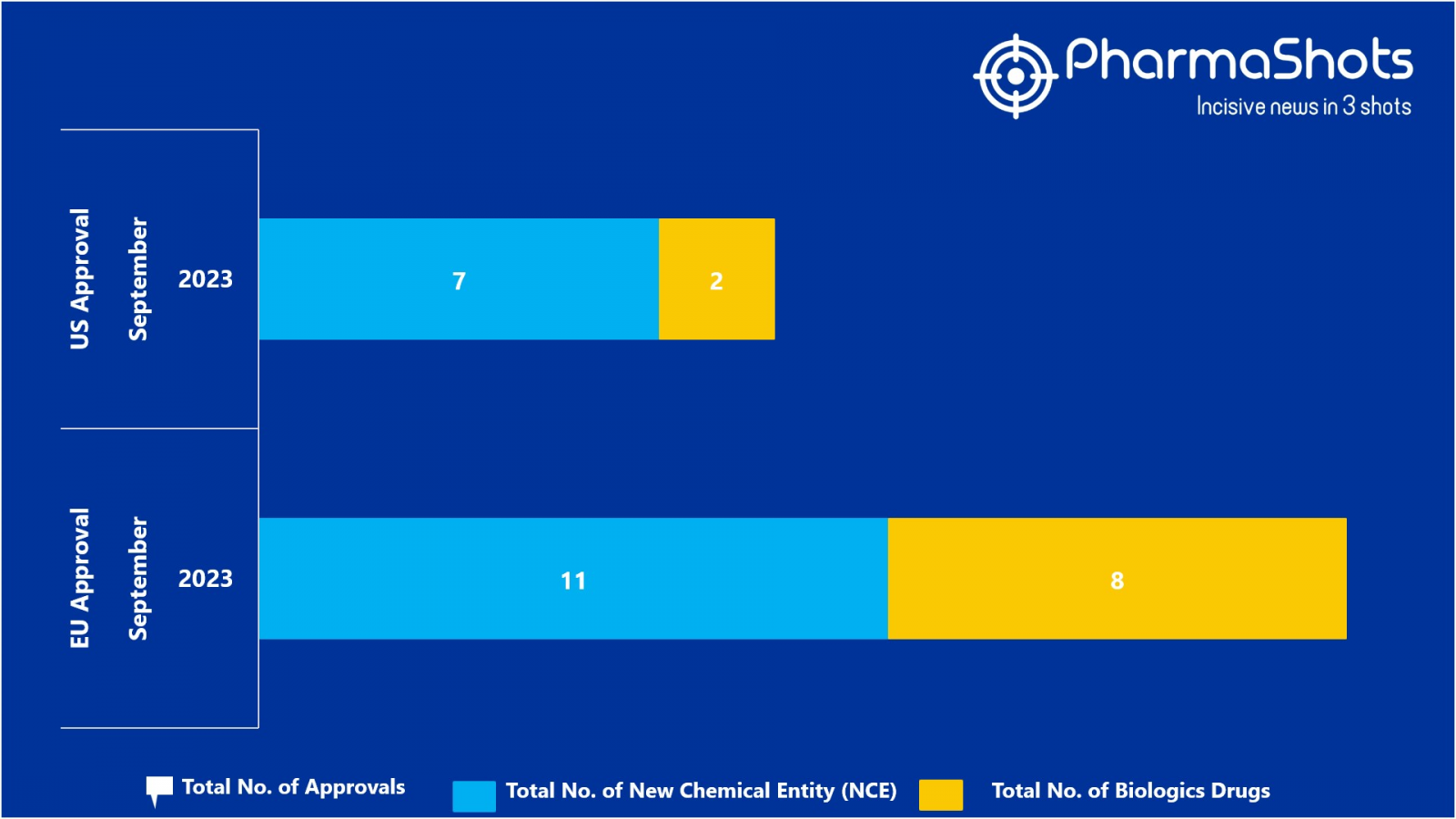
The EMA approved 11 New Chemical Entity (NCE) and 8 Biologic Drugs in September 2023, leading to treatments for patients and advances in the healthcare industry
-
In September 2023, the major highlight drugs were Litfulo (ritlecitinib) for adolescents and adults with s9ere alopecia areata & Orserdu (elacestrant) for ER+, HER2- locally advanced or metastatic breast cancer
-
PharmaShots has compiled a list of a total of 19 new drugs approved by the EMA in September 2023
Givinostat
Active ingredient:N/A Approved: Sept 05, 2023
Company: Italfarmaco Group Disease: Duchenne Muscular Dystrophy
-
The company has submitted the MAA to the EMA for Givinostat to treat DMD & the application is currently under EMA’s review. The MAA was based on the P-III trial (EPIDYS) results of Givinostat (HDAC) inhibitor in 179 ambulant DMD patients aged ≥6yrs.
-
The study met its 1EPs & evaluated functional improvement through mean change from baseline in time to climb four stairs in the target population while a variety of 2EPs were analyzed that showed results consistent with the functional 1EPs. The safety and tolerability profiles were consistent with prior study results
-
The company is currently working with the regulatory authorities to bring the treatment option to patients & offer therapeutic benefits and increase their QoL
2. Astellas Pharma Reports EMA's Validation of Type II Variation for Xtandi (enzalutamide) to Treat Non-Metastatic Hormone-Sensitive Prostate Cancer
Xtandi
Active ingredient: enzalutamide Approved: Sept 12, 2023
Company: Astellas Pharma Disease: Prostate Cancer
-
The submission was based on the P-III trial (EMBARK) evaluating enzalutamide (160mg, qd) + leuprolide or enzalutamide (160mg) as monotx. vs PBO + leuprolide (22.5mg, q12w) in 1068 patients with nmHSPC with high-risk BCR
-
The study met its 1EPs of MFS & showed a reduction in the risk of metastasis or death as assessed by 1EPs of MFS. The overall safety profile was consistent with the known safety profile of each therapy
-
Improvement was also observed in the 2EPs, 37% reduction in risk of metastasis or death in patients with Xtandi monotx., and a 93% reduction in risk of PSA progression in patients treated with enzalutamide + leuprolide or enzalutamide monotx. vs 67% in PBO + leuprolide, 64% reduction in progression risk in starting a new antineoplastic therapy in patients with the combination therapy & 46% in patients with Xtandi monotx.
TransCon PTH
Active ingredient: palopegteriparatide Approved: Sept 15, 2023
Company: Ascendis Pharma Disease: Chronic Hypoparathyroidism
-
The EMA’s CHMP has adopted a positive opinion recommending the approval of TransCon PTH as a parathyroid hormone (PTH) replacement therapy for treating adults with chronic hypoparathyroidism. The EC’s final decision is expected within 67 days after the positive opinion
-
The positive opinion was based on the results from the P-III trial (PaTHway) & P-II (PaTH Forward) trials in adult patients with hypoparathyroidism. Both used the same drug/device combination product as its commercial product for TransCon PTH
-
If TransCon PTH is approved, the first European Union launch is planned in Germany in early 2024
4. argenx Receives the EMA’s CHMP Positive Opinion of Efgartigimod for Generalized Myasthenia Gravis
Vyvgart
Active ingredient: Efgartigimod Approved: Sept 15, 2023
Company: argenx Disease: Generalized Myasthenia Gravis
-
The EMA’s CHMP has recommended approval of efgartigimod (SC) as an add-on to standard therapy for adult patients with gMG who are AChR Ab+. The EC’s decision on MAA is expected within ~60 days
-
The opinion was based on the P-III study (ADAPT-SC) evaluating efgartigimod (SC) vs efgartigimod (IV) in a ratio (1:1) in 110 adult patients in North America, EU & Japan
-
The trial met its 1EPs of noninferiority & showed a mean total IgG reduction of 66.4% vs 62.2% from baseline at 29 Days. The 2EPs were also met which were consistent with efficacy measures from the (ADAPT IV) study, identifying the correlation b/w IgG reduction & clinical benefit. The EC’s decision will be valid for all 27 EU Member States, Iceland, Norway & Liechtenstein
Zilbrysq
Active ingredient: Zilucoplan Approved: Sept 15, 2023
Company: UCB Disease: Generalized Myasthenia Gravis
-
The opinion was based on the P-III study (RAISE) evaluating zilucoplan (0.3mg/kg, SC) vs PBO in a ratio (1:1) in adult patients with AChR Ab+ gMG which showed improvements in gMG-specific efficacy outcomes, significant and clinical difference favoring zilucoplan was observed in the MG-ADL total score change from baseline
-
The 2EPs incl. a change from baseline to 12wk. in QMG, MGC, and MG-QoL15r., significant & clinical difference was seen in QMG total score change from baseline to 12wk. The results were published in the Lancet Neurology journal
-
Zilucoplan is currently under review by PMDA, US FDA, TGA & Health Canada for gMG. Responses from the PMDA & US FDA are expected at the end of Q4 2023; from TGA & Health Canada are expected by H1’24
Kaftrio
Active ingredient: ivacaftor, tezacaftor & elexacaftor Approved: Sept 15, 2023
Company: UCB Disease: Cystic Fibrosis
-
The EMA’s CHMP adopted a positive opinion for the label extension of Kaftrio (ivacaftor/tezacaftor/elexacaftor) in a combination regimen with ivacaftor for CF in patients aged 6-11yrs. who have 1 F508del mutation in CFTR gene
-
If Kaftrio + ivacaftor is approved, it will provide a new treatment option to physicians for young patients to help combat this life-shortening condition shortly
-
Kaftrio + ivacaftor was approved in the EU for CF in patients aged ≥12yrs. who have at least one copy of the F508del mutation in the CFTR gene. Kaftrio showed unprecedented clinical benefit for eligible with CF
Voxzogo
Active ingredient: vosoritide Approved: Sept 15, 2023
Company: BioMarin Disease: Achondroplasia
-
The EMA’s CHMP has adopted a positive opinion recommending marketing authorization to expand the indication for Voxzogo to treat children aged ≥4mos. with achondroplasia whose epiphyses (growth plates) are not closed. The EC’s decision is expected in Q4’23
-
The opinion was based on the P-II trial (111-206) in children aged 4mos.-5yrs. which showed an improvement in height Z-score of ~0.3 standard deviations across all age groups after 1yr. of treatment. The application also incl, longer-term data from the ongoing extension study (111-208)
-
The US FDA’s PDUFA action date for Voxzogo' sNDA is expected on Oct 2023 for children under 5yr. The therapy was approved in the US in children with achondroplasia aged ≥5yr. with open growth plates & in EU aged 2yr.
8. Daiichi Sankyo Receives the EMA’s CHMP Positive Opinion Recommending Approval of Quizartinib for Newly Diagnosed FLT3-ITD Positive AML
Vanflyta
Active ingredient: Quizartinib Approved: Sept 15, 2023
Company: Daiichi Sankyo Disease: FLT3-ITD Positive AML
-
The EMA’s CHMP has recommended the approval of quizartinib for adult patients with newly diagnosed AML i.e., FLT3-ITD+ in combination with standard cytarabine & anthracycline induction and standard cytarabine consolidation CT, followed by quizartinib single-agent maintenance therapy
-
The opinion was based on the P-III trial (QuANTUM-First) of quizartinib + standard induction & consolidation therapy and as maintenance monotx. in a ratio (1:1) in 539 patients aged 18-75yrs.
-
The results showed a 22% reduction in risk of death vs standard CT alone, m-OS (31.9mos.) vs 15.1mos. for patients in the control arm at a median follow-up of 39.2mos. The safety profile was consistent with prior trials with no new safety signals & the results were published in The Lancet
Enhertu
Active ingredient: Trastuzumab deruxtecan Approved: Sept 18, 2023
Company: AstraZeneca and Daiichi Sankyo Disease: Advanced NSCLC
-
The EMA’s CHMP has adopted the positive opinion recommending approval of Enhertu (5.4mg/kg) as monotx. for adult patients with advanced NSCLC whose tumors have an activating HER2 (ERBB2) mutation & require systemic therapy following Pt-based CT with/out immunotherapy
-
The opinion was based on the P-II trial (DESTINY-Lung02) of Enhertu in a ratio (2:1) in 152 patients at multiple sites, incl. Asia, EU & North America, showed ORR (49.0%), DCR (93.1%), CR (1.0%), PR (48.0%), m-DoR (16.8mos.) & the results were presented at IASLC 2023 & published in the Journal of Clinical Oncology
-
The safety profile was consistent with prior clinical trials with no new safety signals. Enhertu (5.4mg/kg) was approved in 50+ countries for unresectable or metastatic HER2+ breast cancer
Keytruda
Active ingredient: pembrolizumab Approved: Sept 18, 2023
Company: Merck Disease: Non-Small Cell Lung Cancer
-
The EMA’s CHMP adopted a positive opinion recommending approval of Keytruda for the adjuvant treatment of adults with NSCLC who are at high risk of recurrence following complete resection and Pt-based CT. The EC’s final decision is expected in Q4’23
-
The opinion was based on results from the P-III trial (KEYNOTE-091) evaluating Keytruda. The trial was conducted in collaboration with EORTC and ETOP which showed a significant improvement in DFS
-
Keytruda, an anti-programmed death receptor-1 (PD-1) therapy was approved in Jan 2023 as monotx. for adjuvant treatment following surgical resection and Pt-based CT for adult patients with stage IB (T2a ≥4 centimeters), II, or IIIA NSCLC in the US
Litfulo
Active ingredient: ritlecitinib Approved: Sept 19, 2023
Company: Pfizer Disease: Alopecia Areata
-
The EC has granted marketing authorization to Litfulo for adults & adolescents aged ≥12yrs. with sev. alopecia areata. The approval was based on the (ALLEGRO) trial program incl. P-IIb/III trial (ALLEGRO) of Litfulo vs PBO in 718 patients
-
The results showed that 13.4% of adults and adolescents achieved ≥90% scalp hair coverage (SALT ≤10) after 24wks. with Litfulo (50mg) vs 1.5% with PBO, PGI-C response was measured, 49.2% vs 9.2% reported a response of “moderate” to “great” improvement in alopecia areata at 24wks.
-
The marketing authorization is valid in all 27 EU member states, Iceland, Liechtenstein & Norway. Litfulo is the first and only treatment to selectively inhibit JAK3 & tyrosine kinase expressed in the hepatocellular carcinoma family of kinases
Apretude
Active ingredient: cabotegravir Approved: Sept 19 2023
Company: ViiV Healthcare Disease: HIV
-
The approval was based on the results from two P-IIb/III active-controlled studies (HPTN 083 & 84) evaluating cabotegravir LA vs oral FTC/TDF tablets (200 mg/300 mg) for PrEP in HIV- men who have sex with men, transgender women and cisgender women at increased risk of sexually acquired HIV
-
The results showed that cabotegravir LA was superior to daily oral FTC/TDF tablets & experienced a 69% & 90% lower rate of HIV acquisition vs FTC/TDF tablets in (HPTN 083) & (HPTN 084) trial
-
Cabotegravir LA was approved for use in the US, Australia, South Africa & number of other countries, as Apretude for PrEP while the submission to other regulatory agencies is on-going
Inaqovi
Active ingredient: decitabine and cedazuridine Approved: Sept 19, 2023
Company: Otsuka and Astex Disease: Acute Myeloid Leukaemia
-
The EC has approved Inaqovi (oral decitabine and cedazuridine) as monotx. for adult patients with newly diagnosed AML who are ineligible for standard induction CT. The approval was based on the P-III trial (ASCERTAIN) evaluating fixed-dose combination vs decitabine (IV) in a ratio (1:1) in 89 AML patients
-
The study met its 1EPs i.e., the fixed-dose combination showed PK exposure equivalence of 99.64% to IV decitabine given at 20 mg/m2 for 5 days using a two-cycle, cross-over study design. The secondary results showed a m-OS of 7.9mos. & CR rate of 21.8% at 7.95mos. median follow up
-
The safety was consistent with those anticipated for IV decitabine. The EC’s decision will be valid to EEA incl. the EU member states, Iceland, Liechtenstein & Norway\
Tevimbra
Active ingredient: tislelizumab Approved: Sept 19, 2023
Company: BeiGene Disease: Esophageal Squamous Cell Carcinoma
-
The EC has approved Tevimbra as a monotx. for adult patients with unresectable, LA, or metastatic ESCC after prior Pt-based CT. The approval was based on the P-III study (RATIONALE 302) evaluating Tevimbra vs CT in 513 patients from 132 research sites in 11 countries in the EU, Asia & North America
-
The trial met its 1EPs in the ITT population with a significant clinical survival benefit, m-OS (8.6 vs 6.3mos.). The safety profile was consistent with prior trials
-
Additionally, the US FDA has accepted for review a BLA of tislelizumab as a 1L treatment for unresectable, recurrent, LA, or metastatic ESCC with an anticipated PDUFA date in H2’24. Tislelizumab received ODD from the US FDA for previously untreated advanced or metastatic ESCC
Orserdu
Active ingredient: elacestrant Approved: Sept 20, 2023
Company: Menarini Group and Stemline Therapeutics Disease: Breast Cancer
-
The EC has approved Orserdu as a monotx. for postmenopausal women & men, with ER+, HER2-, LA or mBC with an activating ESR1 mutation who have disease progression following one line of endocrine therapy incl. a CDK 4/6 inhibitor
-
The approval was based on the P-III trial (EMERALD) evaluating elacestrant as monotx. vs SoC in 478 patients which showed a significant PFS, m-PFS (3.8 vs 1.9mos.) in the group of patients whose tumors had ESR1 mutations, 45% reduction in risk of progression or death
-
In a post hoc subgroup analysis, the duration of prior CDK4/6i treatment was associated with longer PFS on elacestrant, m-PFS (8.6 vs 1.9mos.) for patients with ESR1 mutations treated with CDK4/6i for ≥12mos., 59% reduction in risk of progression or death
Enrylaze
Active ingredient: crisantaspase Approved: Sept 22, 2023
Company: Jazz Disease: ALL & LBL
-
The EC has granted marketing authorization for Enrylaze for use as a component of a multi-agent chemotherapeutic regimen to treat ALL & LBL in adult & pediatric patients aged ≥1mos.
-
The approval was based on the P-II/III trial (conducted in collaboration with Children's Oncology Group) evaluating Enrylaze (administered as IV infusions and IM inj.) in 228 pediatric & adult patients, showed that patients treated with JZP458 as IV maintained NSAA ≥0.1 U/mL (89.8%) at 48hrs. after a dose & 40% at 72hrs. post-dose
-
The IM administration of JZP458 (25/25/50mg/m2 MWF) achieved sustained asparagine activity in 95.9% at 48hrs. & 89.8% at 72hrs. post-dose. Enrylaze was approved as Rylaze in the US & Canada
Tepkinly
Active ingredient: epcoritamab Approved: Sept 26, 2023
Company: AbbVie and Genmab Disease: Diffuse Large B-cell Lymphoma
-
The EC has granted conditional marketing authorization for Tepkinly as a monotx. for the treatment of adult patients with r/r DLBCL after two or more lines of systemic therapy
-
The approval was based on the results from the P-I/II trial (EPCORE NHL-1) evaluating Tepkinly in patients with r/r LBCL incl. subtype DLBCL. The results showed ORR (62%) and a CR rate (39%), m-DoR was 15.5mos. & also demonstrated a manageable safety profile across the LBCL patient cohort incl. the DLBCL patient population
-
Tepkinly, an IgG1-bispecific Ab that has been developed using Genmab's DuoBody technology. Epcoritamab was approved in the US under the brand name Epkinly in May 2023 for r/r DLBCL
Ryeqo
Active ingredient: relugolix, estradiol & norethisterone acetate Approved: Sept 28, 2023
Company: Gedeon Richter and Sumitomo Pharma Disease: Endometriosis
-
The EMA’s CHMP has adopted a positive opinion recommending the approval of a Type II Variation application for Ryeqo (relugolix 40 mg, estradiol 1.0 mg, and norethisterone acetate 0.5 mg) to treat symptomatic treatment of endometriosis in women with a history of previous medical or surgical treatment for their endometriosis. The EC’s final decision on MAA is expected in the coming months
-
The application was based on the two, 24wk. clinical studies (SPIRIT 1 & 2) in 1200+ women with mod. to sev. pain associated with endometriosis, as well as the 80wk. OLE study of Ryeqo
-
Ryeqo was approved by the EMA for mod. to sev. symptoms of uterine fibroids in adult women of reproductive age. The decision will be valid to all member states of the EEA
GRN163L
Active ingredient: Imetelstat sodium Approved: Sept 29, 2023
Company: Geron Disease: Myelodysplastic Syndromes
-
The MAA was based on the P-III trial (IMerge) evaluating imetelstat vs PBO which showed that the 1EPs of 8wk. transfusion independence was higher with imetelstat vs PBO, and median TI duration was 1yr. for imetelstat 8wk. TI responders, mean Hb levels were increased over time vs PBO
-
Significant & clinical efficacy results were achieved across MDS subgroups irrespective of ring sideroblast status, baseline transfusion burden & IPSS risk category, PRO data showed a sustained improvement in fatigue
-
The MAA is now under CHMP’s review under the centralized procedure valid in all 27 EU member states, Iceland, Norway & Liechtenstein while the review of the MAA is expected to be ~14mos.
Related Post: Insights+: EMA Marketing Authorization of New Drugs in August 2023
Tags

Neha is a Senior Editor at PharmaShots. She is passionate and very enthusiastic about recent updates and developments in the life sciences and pharma industry. She covers Biopharma, MedTech, and Digital health segments along with different reports at PharmaShots. She can be contacted at connect@pharmashots.com.













